"Dr. Ball," she asked, "what would bethe perfect cancer treatment?"He smiled, "Do you mean 'the Magic Bullet'?"
He stands dressed in a green scrub suit, working hard over his anesthetised patient, and he looks like any other surgeon, but he's not a surgeon. His hands get tired and begin to cramp after hours of drilling bone and suctioning marrow. He knows that tonight he'll be spending many more hours in the lab with this patient's vital fluid, treating it and washing it, treating it and washing it, and then slowly freezing it and carefully storing it until the time comes for his patient to need it again.
A new nurse, unfamiliar with the technique which outwardly appears mundane and nonthreatening, by accident nearly knocks over the stainless steel beaker holding the bloodlike fluid. "Be careful," he warns in a quiet voice, hardly revealing his concern, "her life depends on that stuff."
The nurse looks at him, confused by his matter-of-fact tone delivering such a menacing message. "Are you serious?" she asks.
"Yes, very."
His patient is a young mother from the Midwest, a victim of bone marrow cancer, acute myelogenous leukemia. She and her husband have traveled from Kansas City, Missouri to Hanover, New Hampshire in order for her to receive the treatment that may prove to be curative monoclonal antibodies. Specific, one-of-a-kind monoclonal antibodies developed by Dr. Edward "Ted" Ball, a specialist in diseases of the blood.
There are only four institutions in the United States doing research in monoclonal antibodies as treatment for myeloid leukemia. Ted Ball's team at the Dartmouth-Hitchcock Medical Center has the highest success rate. "A lot of people even at 'Hitch' don't know the extent of our contribution," Ball says, "but we have the most effective monoclonal antibodies in the world. Somebody might challenge that if it were made public, but the fact is, right now, ours are the best."
He is a tall man, with dark hair and blue eyes who looks far too young to be at the forefront of leukemia research. Ball received his medical degree from Case Western University, then interned at Hartford Hospital in Hartford, Connecticut. He returned to Case Western for his residency in hematology and as his knowledge of the terrible disease expanded, it began to unveil itself, showing its meanness and perverted physiology, challenging him. "It's extremely fascinating," he says, then as if to say it with clearer meaning, he repeats the same words, "a fascinating disease."
Six years ago in Cleveland, Ohio, at Case Western University, Ball began research in monoclonal antibodies. He had just finished his residency in hematology and was looking at ways to improve treatment of patients with leukemia. The conventional approaches of chemotherapy and radiation were not improving survival rates. Everyone in the field was looking for a new drug or combination of drugs that would be more poisonous to leukemia cells and less poisonous to healthy cells. Ted Ball looked in a different direction.
A new technology was on the horizon, genetic manufacturing, and Ball saw the opportunity to develop a leukemia killer even more specific thapoison. A killer exactly like one of the body's own natural defenders an antibody able to recognize and destroy leukemia cells, but leave other cells unharmed. If an antibody could be "programed" to recognize a dangerous leukemia cell, and if that antib ody could be genetically manufactured in quantity, then perhaps a "magic bullet" against leukemia could actually exist.
There were a lot of "ifs" to overcome, but at Case Western, Ball developed his first monoclonal antibody against leukemia. Years later, after moving to the Dartmouth Medical School, and funded by a federal grant of $250,000, he developed a second antibody.
The process of producing monoclonal antibodies is dubbed by the scie nce community as "hybridoma technology." It requires three things: leukemia antigen, a mouse, and a dish of living, specially made cells. Add years of trial and error. The thing about this kind of research is time investment. It doesn't happen that one day in the lab a researcher looks down at a dish of cells and exclaims, "Voila! leukemia killers." "It takes year after year of building a picture to realize what you have," says Ball.
First, leukemia cells, like all cells, have embedded in their molecular framework a chemical pattern called "antigen." Antigens have a shape and chemical property as recognizable as a fingerprint. An antibody will discover the "fingerprint" it is programmed to find and immediately bind itself to the antigen.
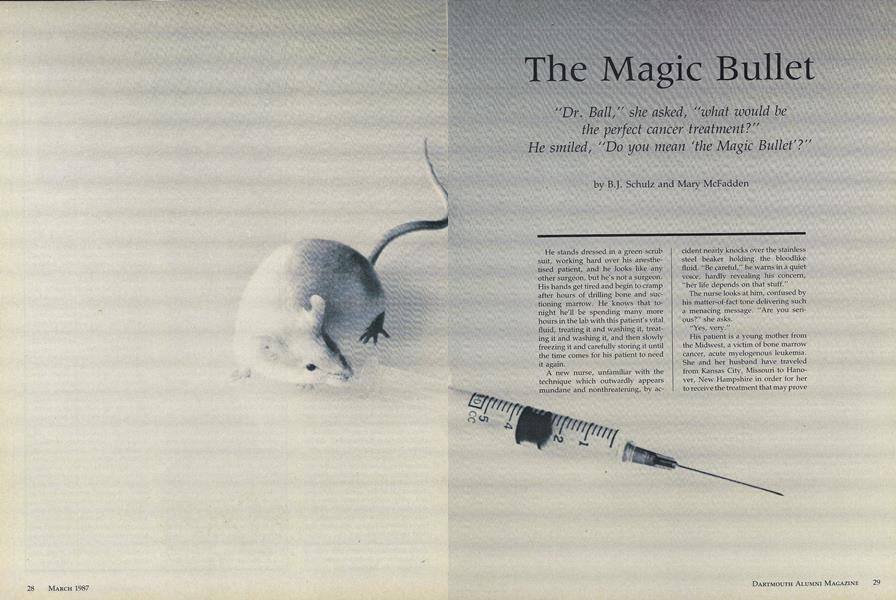
Second, mice are unlucky enough to have spleens particularly suited to make antibodies. After leukemia gen is injected into the mouse spleen, it starts programming antibodies against the antigen. When its spleen is at full production, the mouse is sacrificed and its spleen is liquified.
antiThird, the spleen fluid is then poured into a dish of special cells which take within them the mouse spleen's method for making antigen. In essence the mouse cell and the special cell join together making a hybrid. These special cells start turning out ant ibodies in large quantity. The product which results when the spleen cells are mixed with the special cells is called "hybridoma," which is how the technology gets its name.
Because the antibody is specificallyprogrammed against a certain type of leukemia, a patient's leukemia cells must first be tested against the antibody. If his or her leukemia cells display the antigen that the programmed antibodies are designed to find, then the antibody will bind to it. In the process of binding to the antigen, the ant ibody actually affixes itself to the leukemia cell and either destroys it at once or renders it helpless against the body's other defense systems. It is a requirement, then, that the patient have leukemia cells with antigens that are recognizable by Ball's monoclonal antibodies.
If the patient's leukemia cells display the right antigen, then the process of treatment will occur when the disease is at remission. Under anesthesia the patient's bone marrow is "harvested" in sufficient quantity to replace totally the amount he or she would require to survive. "Replace" is the correct word here because the next step in the treatment is the complete destruction of the patient's bone marrow by a combination of conventional chemotherapy and radiation therapy. The goal is to destroy every single leukemia cell in the patient's body.
Immediately after harvesting, the bone marrow cells are treated with the monoclonal antibodies. It is a timeconsuming procedure which continues well into the night. There are only two places in the world that a patient's bone marrow can be treated with Ball's antibodies Dartmouth, and Scripps Clinic and Research Foundation in southern California' where Ball has sent his product to be prepared. These antibodies are "among the most effect ive in the world," he explains. After treatment, the bone marrow is given back to the patient intravenously. The cells migrate to their natural areas and begin their job of making normal blood cells, without leukemia.
"It's an innovative approach to the treatment of cancer that offers the chance for a prolonged remission," Ball states with little inflection in his voice, letting the meaning fall on the listener with only its semantic weight. "We're working toward doing the same thing in lung cancer, lymphoma and other types of malignancies."
In short order, he has just summed up one of the most exciting developments in medical research and the treatment of cancer. It is very possible that in the next century, cancer, and perhaps exotic infections, will be controlled or cured by genetically manufactured monoclonal antibodies. Imagine a time when antibodies against a particular patient's cancer could be manufactured like an arrangement of magic bullets.
Dr. Ted Ball transfers bone marrow cells intoa test tube. The cells will then be added tomonoclonal antibodies obtained from the purified ascites fluid of mice, producing "TheMagic Bullet."
Britt Schulz lives in South Pomfret, Vermont and is the executive editor of Upper Valley Magazine. Mary McFadden is afreelance writer from Lebanon, NH. Thearticle was reprinted with permission from Upper Valley Magazine, Novem-ber/December 1986.
 View Full Issue
View Full Issue
More From This Issue
-
 Feature
Feature"These Children Are the Future"
March 1987 By Shelby Grantham -
 Cover Story
Cover StoryPassing With A Roll Of The Dice
March 1987 By Jay Heinrichs -
 Feature
FeatureOne Question for Mr. Frost
March 1987 By Philip Booth '47 -
 Article
ArticleDartmouth Authors
March 1987 -
 Class Notes
Class Notes1983
March 1987 By Ken Johnson -
 Article
ArticleSenior Epiphany
March 1987 By Lesley Barnes '87
Features
-
 Feature
Feature1960 ALUMNI FUND STATEMENT
December 1960 -
 Feature
FeatureFour Views of Educational Opportunity at Convocation Opening the 203rd Year
NOVEMBER 1972 -
 Feature
FeatureArt Carpenter
APRIL 1973 -
 Cover Story
Cover StoryPEAVEY HEAD
MARCH | APRIL 2014 -
 Feature
FeatureWarner's 41 Dramatic Years
MAY 1969 By MARGARET BECK McCALLUM -
 Feature
FeatureAnother Day, Another Dollar
March 1975 By V.F.Z.